ABM-1310 is highly potent and selective BRAF inhibitor with an excellent cell permeability and brain penetration. The compound was designed to address majority of BRAF V600E mutated cancers, which happens in about 8% total cancers, such as colorectal cancer (which has a high percentage of cancer stem cell subpopulation), melanoma drug resistance and brain metastasis, and glioma (current drugs are limited by poor brain penetration), as potentially the best-in-class. It was granted clearance of the Investigational New Drug (IND) application to proceed with the first-in-human clinical trial in November 2019 in the USA. (Clinical trial information: NCT04190628) More information about ABM’s ongoing ABM-1310 trial is available at www.ClinicalTrials.gov and on the company website at www.abmtx.com. ABM-1310 also got IND approval by NMPA in China in November 2021.
ABM-168 is a highly potent allosteric MEK inhibitor with good cell permeability and brain penetration. While MEK inhibitors as a class present a low therapeutic window due to intrinsic cytotoxicity, Our approach is to combine ABM-168 with another oncogenic driver inhibitor such as a BRAF inhibitor. The compound received IND approval by FDA in Oct. 2022.
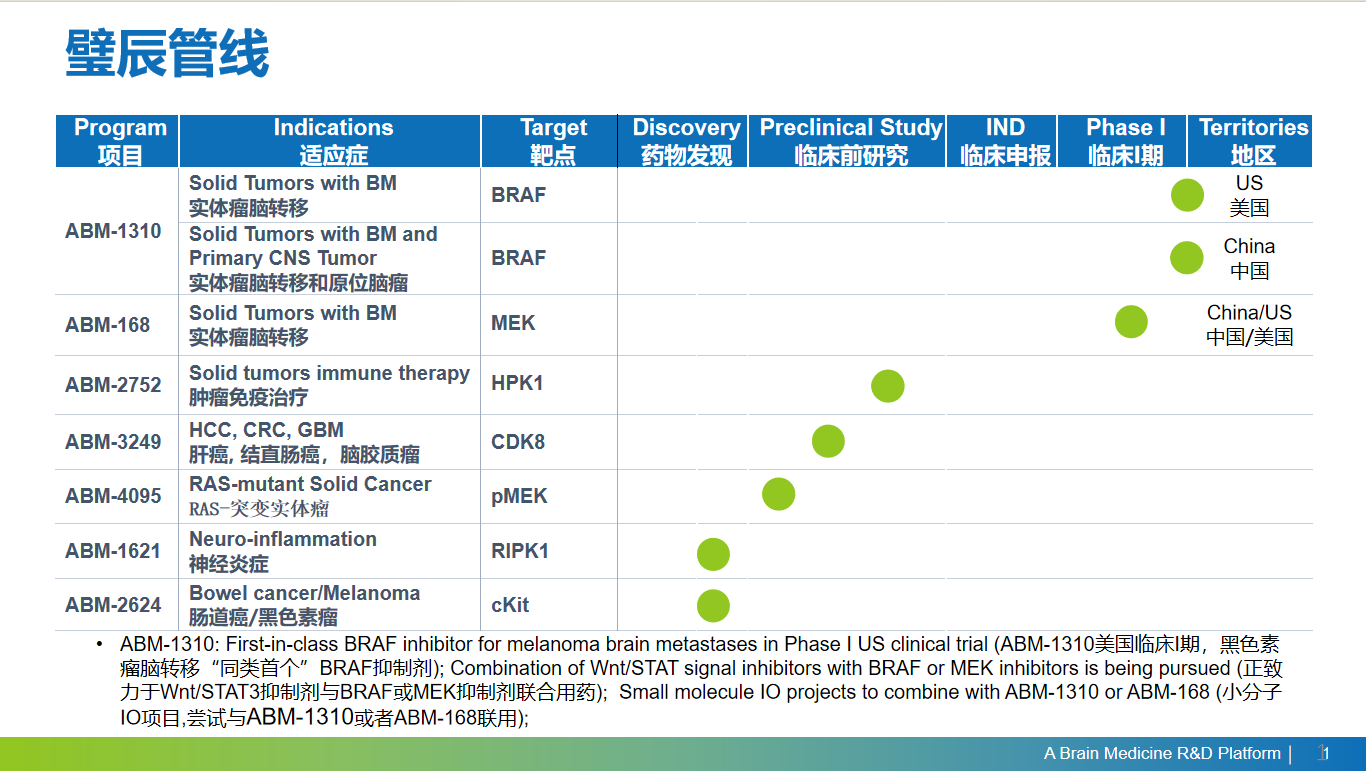
ABM-2752 is a potent allosteric HPK1 inhibitor which is minimally affected by ATP concentrations. HPK1 is a serine/threonine kinase which negatively regulates T-Cell activities, therefore, an effective inhibition of HPK1 kinase could activate the immune response to cancer. ABM-2752 has demonstrated excellent efficacies in several syngeneic mouse models such as CT26, MC38 and B16F10, single or in combination with a PD-1 antibody. The compound is currently in clinical-enabling studies.
Several other programs are in progress, these compounds targeting Src/STAT3 and Wnt/b-catenin signal pathways to address cancer metastasis and drug-resistance. These compounds are expected to have synergistic effects with MAPK inhibitors to target tumor growth and cancer metastasis.

